- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
IB DP Physics: HL復習筆記12.2.3 Deviations from Rutherford Scattering
Deviations from Rutherford Scattering
- In the Rutherford scattering experiment, alpha particles are fired at a thin gold foil
- Some of the alpha particles are found to come straight back from the gold foil
- This indicates that there is?electrostatic repulsion?between the alpha particles and the gold nucleus
- At the point of closest approach,?r, the repulsive force reduces the speed of the alpha particles to zero momentarily
- At this point, the initial kinetic energy of an alpha particle,?Ek, is?equal?to electric potential energy,?Ep
- Then the alpha particle will be electrostatically repelled

The closest approach method of determining the size of a gold nucleus
- This experiment assumes that the alpha particles are?only?interacting through?electrostatic repulsion
Deviations from Rutherford Scattering
- If the energy of the alpha particles exceeds?27.5 - 28 MeV, then they will be close enough to?interact?with the nucleus via the?strong nuclear force
- The Rutherford formula describes this as it states that as the angle of scattering angle increases, the number of alpha particles scattered at that angle sharply decreases
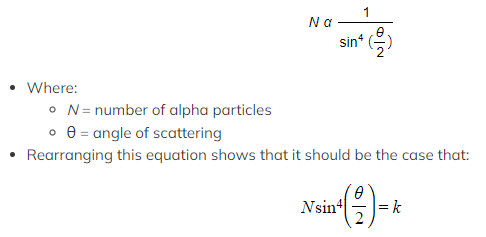
- Experimental data bears this out, suggesting that the Rutherford formula is correct
- A number of assumptions are made in deriving this formula, with the main one being that the only force we need to consider is the electric force
- Deviations from Rutherford scattering are evidence of the?strong nuclear force
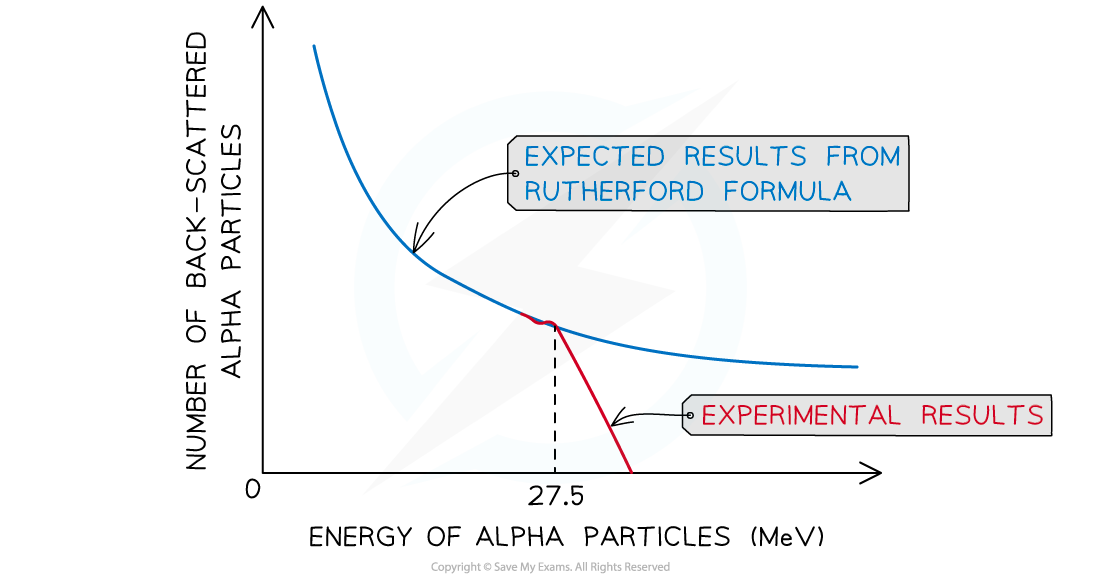
The observed back-scattering from alpha particles strongly deviates from the predicted relationship based only on electromagnetic repulsion at 27.5 MeV
Worked Example

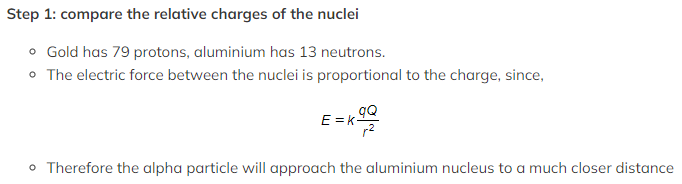 Step 2: consider what causes deviation from Rutherford scattering
Step 2: consider what causes deviation from Rutherford scattering
-
- Deviations from Rutherford scattering occur when forces apart from the electric force between the nuclei are at play
- At much smaller distances the effect of the strong nuclear force will be felt
Step 3: deduce the solution
-
- The alpha particles gets closer to the aluminium nucleus, and the strong nuclear force affects them
- More deviation will be seen with aluminium foil than with gold foil
Exam Tip
- The greatest deviations from Rutherford scattering occur when the energy of the alpha particles is high and the radius of the target nuclei is small (meaning it has a small nucleon number)
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









