- 翰林提供學(xué)術(shù)活動、國際課程、科研項目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
IB DP Chemistry: SL復(fù)習(xí)筆記5.1.3 Calorimetry
Calorimetry
Measuring enthalpy changes
- Calorimetry?is a technique used to measure changes in enthalpy of chemical reactions
- A?calorimeter?can be made up of a?polystyrene drinking cup, a?vacuum flask?or?metal can
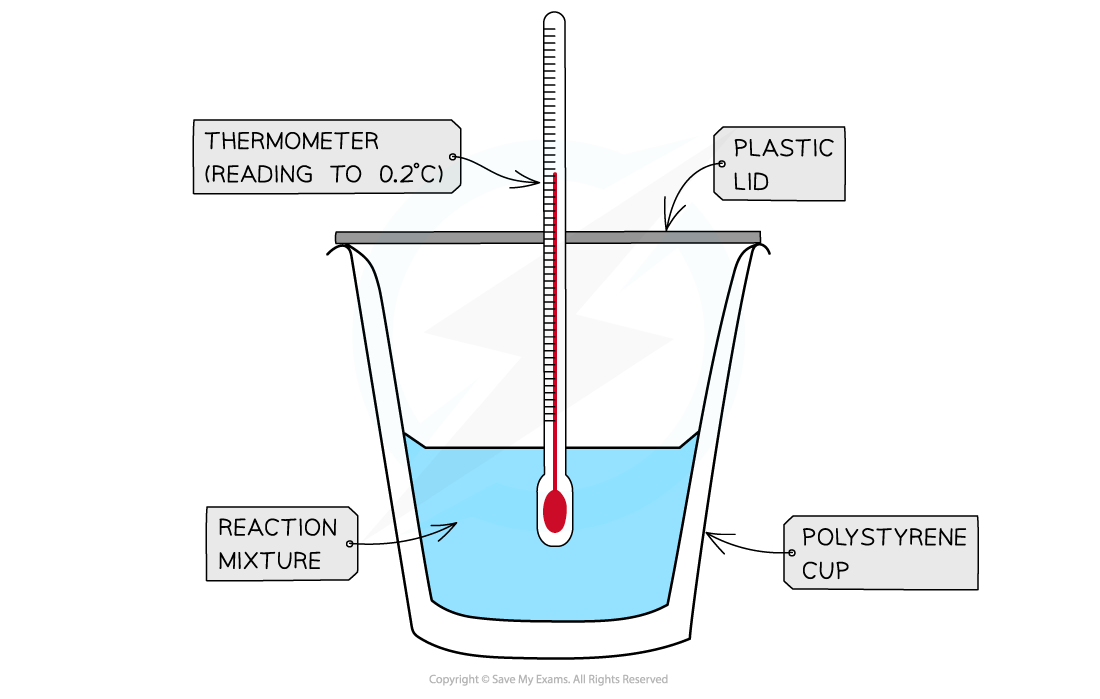
A polystyrene cup can act as a calorimeter to find enthalpy changes in a chemical reaction
- The energy needed to raise the temperature of 1 g of a substance by 1 K is called the?specific heat capacity?(c) of the liquid
- The?specific heat capacity?of water is 4.18 J g-1?K-1
- The energy transferred as heat can be calculated by:
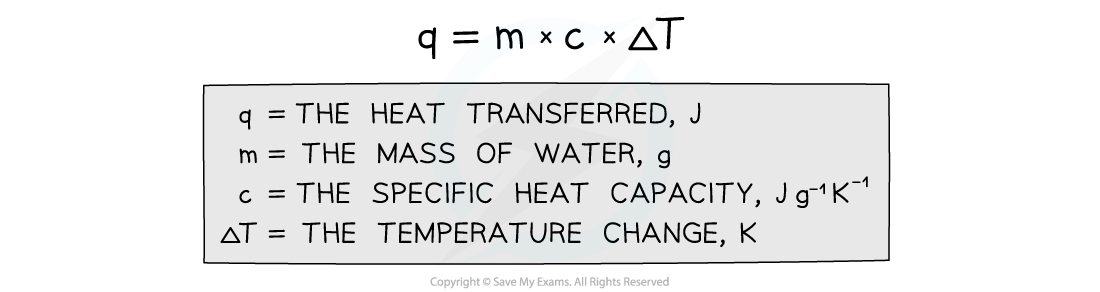
Equation for calculating energy transferred in a calorimeter
Worked Example
The energy from 0.01 mol of propan-1-ol was used to heat up 250 g of water. The temperature of the water rose from 298K to 310K (the specific heat capacity of water is 4.18 J g-1?K-1.Calculate the enthalpy of combustion.
Answer:
Step 1:?q = m x c x ΔT
m?(of water) = 250 g
c?(of water) = 4.18 J g-1?K-1
ΔT?(of water) = 310 - 298 K = 12 K
Step 2:?q = 250 x 4.18 x 12= 12 540 J
Step 3:? This is the energy released by 0.01 mol of propan-1-ol
Total energy? ? ΔH = q?÷ n = 12 540 J ÷ 0.01 mol = 1 254 000 J mol-1
Total energy =?- 1254 kJ mol-1
Exam Tip
There's no need to convert the temperature units in calorimetry as the change in temperature in?oC is equal to the change in temperature in K
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









