- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
IB DP Chemistry: SL復習筆記8.2.7 Strong & Weak Acids & Bases
Strong & Weak Acids & Bases
Strong acids
- A?strong acid?is an acid that?dissociates?almost?completely?in aqueous solutions
- HCl (hydrochloric acid), HNO3?(nitric acid) and H2SO4?(sulfuric acid)
- The position of the equilibrium is so far over to the?right?that you can represent the reaction as an irreversible reaction
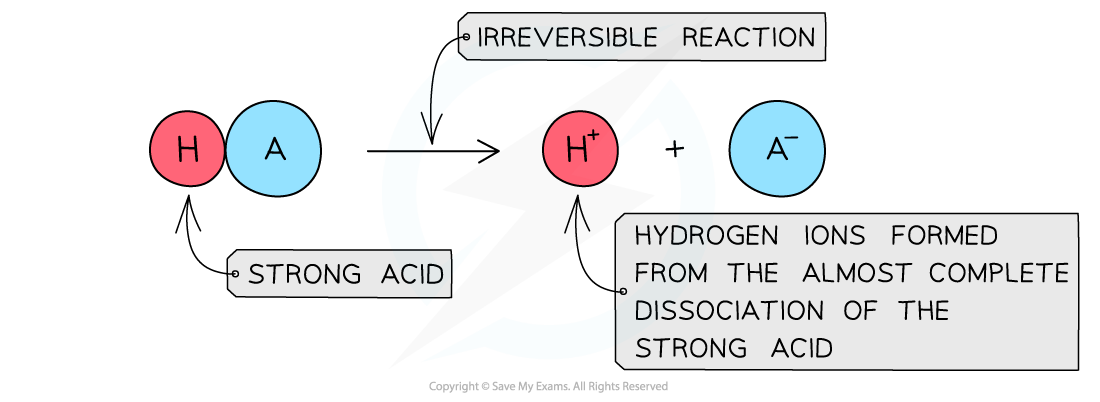
The diagram shows the complete dissociation of a strong acid in aqueous solution
- The solution formed is?highly acidic?due to the high concentration of the H+/H3O+?ions
- Since the?pH?depends on the concentration of H+/H3O+?ions, the pH can be calculated if the concentration of the strong acid is known
 pH is the negative log of the concentration of H+/H3O+?ions and can be calculated if the?concentration of the strong acid is known using the stoichiometry of the reaction
pH is the negative log of the concentration of H+/H3O+?ions and can be calculated if the?concentration of the strong acid is known using the stoichiometry of the reaction
Weak acids
- A?weak acid?is an acid that?partially?(or incompletely)?dissociates?in aqueous solutions
- Eg. most organic acids (ethanoic acid), HCN (hydrocyanic acid), H2S (hydrogen sulfide) and H2CO3?(carbonic acid)
- The position of the equilibrium is more over to the?left?and an equilibrium is established
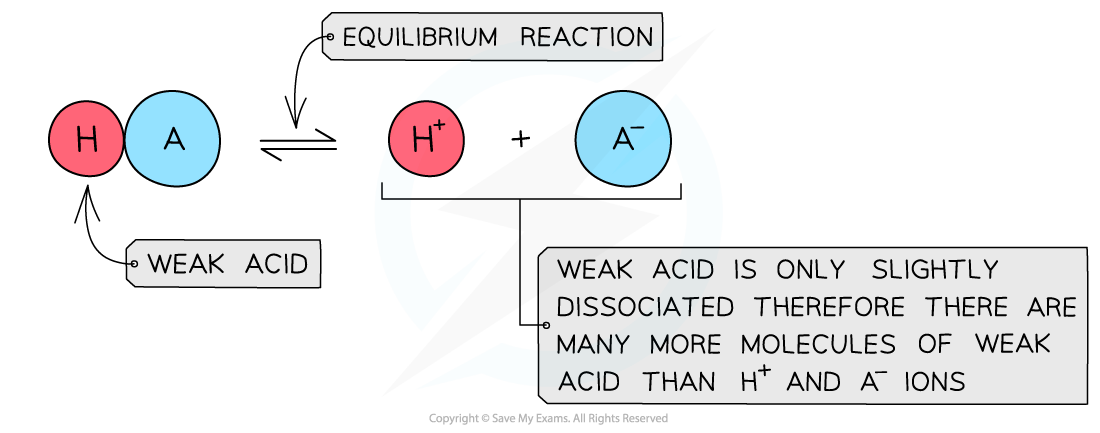
The diagram shows the partial dissociation of a weak acid in aqueous solution
- The solution is?less acidic?due to the lower concentration of H+/H3O+?ions
- Finding the pH of a weak acid requires using the acid dissociation constant, Ka?but this not required at Standard Level, but only at Higher Level and is covered in Topic 18
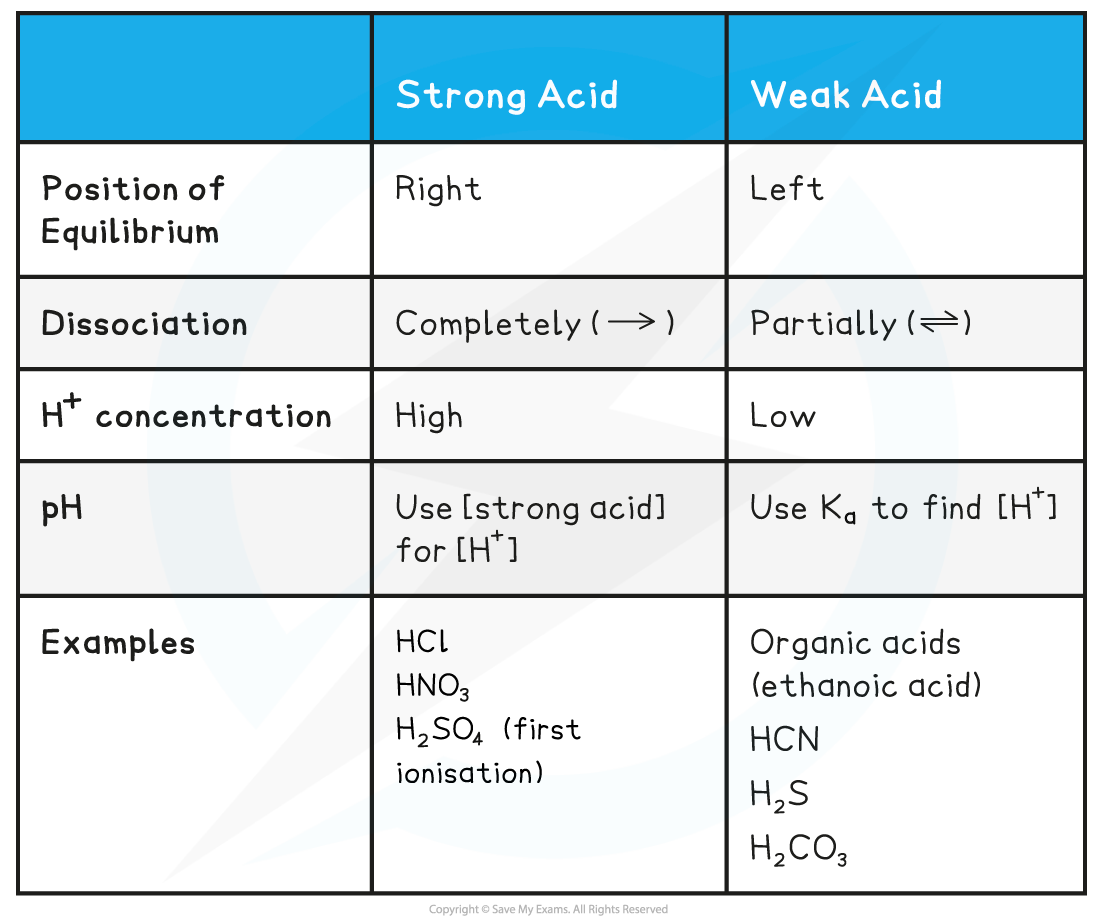
Acid & Equilibrium Position Table
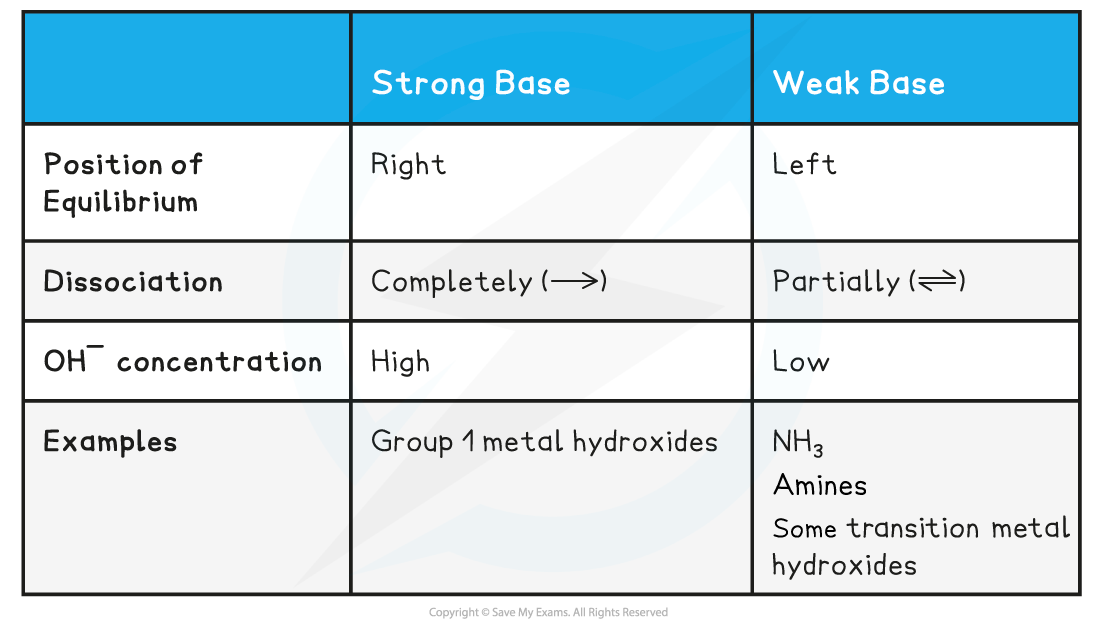
Strong bases
- A?strong base?is a base that dissociates almost completely in aqueous solutionsE.g. group 1 metal hydroxides such as NaOH (sodium hydroxide)
- The position of the equilibrium is so far over to the right that you can represent the reaction as an irreversible reaction
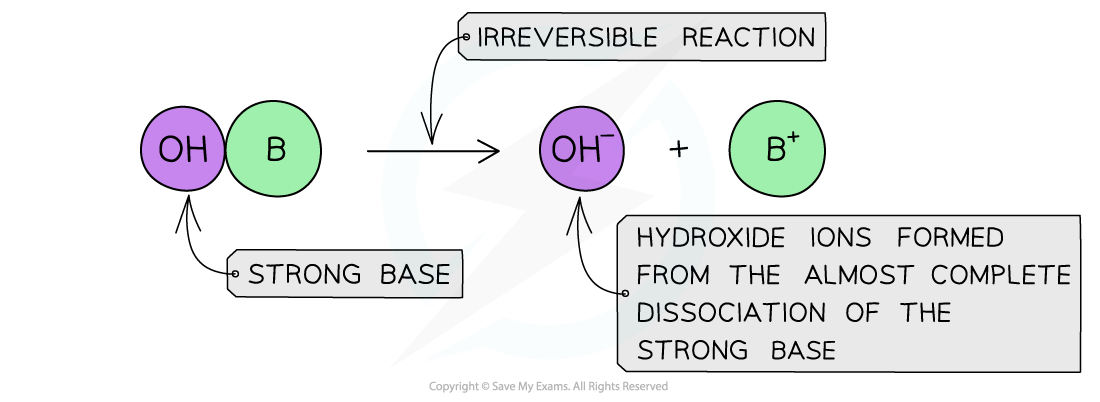 The diagram shows the complete dissociation of a strong base in aqueous solution
The diagram shows the complete dissociation of a strong base in aqueous solution
- The solution formed is highly basic due to the high concentration of the OH-?ions
Weak bases
- A?weak base?is a base that?partially?(or incompletely)?dissociates?in aqueous solutions
- NH3?(ammonia), amines and some hydroxides of transition metals
- The position of the equilibrium is more to the?left?and an equilibrium is established
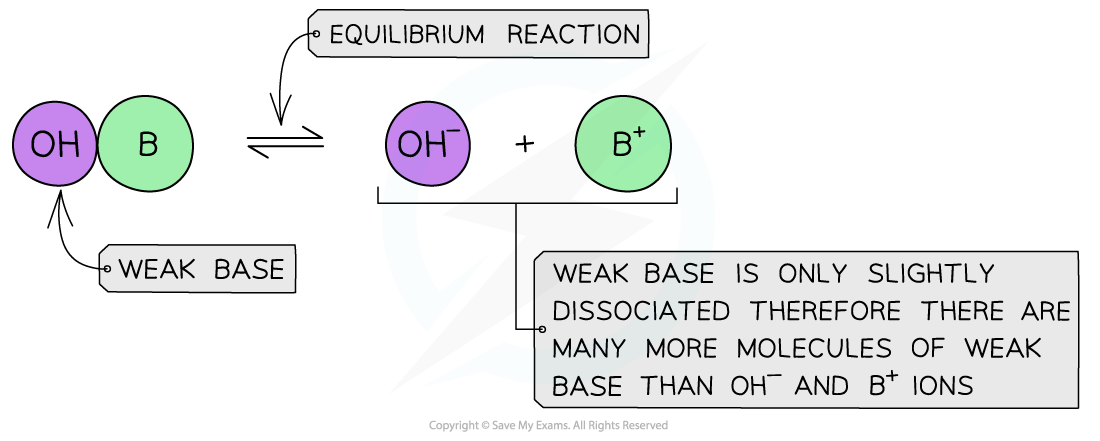
The diagram shows the partial dissociation of a weak base in aqueous solution
- The solution is?less basic?due to the lower concentration of OH-?ions
Base & Equilibrium Position Table
Conjugate Pairs & Acid-Base Strength
- The conjugate base of HCl is the chloride ion, Cl-, but since the reverse reaction is virtually non-existent the chloride ion must be a very weak conjugate base
HCl (g) → H+?(aq)? +? ?Cl-?(aq)
acid? ? ? ? ? ? ? ? ? ? ? ? ? conjugate base
- In general?strong acids?produce?weak conjugate bases?and?weak acids?produce?strong conjugate bases
- A strong base is also fully ionized and is a good proton acceptor
- For example the hydroxide ion is a strong base and readily accepts protons:
OH-?(aq)+??H+?(aq)? ?? H2O (l)
- The conjugate acid of the hydroxide ion is water, which is a weak conjugate acid
- In general?strong bases?produce?weak conjugate acids
Exam Tip
Hydrogen ions in aqueous solutions can be written as either as H3O+?or as H+?however, if H3O+?is used, H2O should be included in the chemical equation:?HCl(g) → H+(aq) + Cl-(aq)? ?OR?HCl(g) + H2O(l) → H3O+(aq) + Cl-(aq)?Some acids contain two replaceable protons ( called 'dibasic') – for example, H2SO4?(sulfuric acid) has two ionisations: H2SO4?acts as a strong acid:?H2SO4?→ H+?+ SO4-HSO4-?acts as a weak acid:?HSO4-?? H+?+ SO42-The second ionisation is only partial which is why the concentration of 1?mol dm-3?sulfuric acid is not 2?mol dm-3?in H+?ions?Also, don't forget that the terms?strong?and?weak?acids and bases are related to the?degree of dissociation?and not the?concentration.The appropriate terms to use when describing?concentration?are?dilute?and?concentrated.
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









