- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
IB DP Chemistry: SL復習筆記11.1.2 Mass Spectrometry
Determining Molecular Mass
- When a compound is analysed in a mass spectrometer, vaporised molecules are bombarded with a beam of high-speed electrons
- These knock off an electron from some of the molecules, creating?molecular ions:

- The relative abundances of the detected ions form a?mass spectrum: a kind of molecular fingerprint that can be identified by computer using a spectral database
- The peak with the highest?m/e?value is the molecular ion (M+) peak which gives information about the?molecular?mass?of the compound
- This value of m/z is equal to the?relative molecular mass?of the compound
The M+1 peak
- The [M+1]?peak is a smaller peak which is due to the natural abundance of the isotope?carbon-13
- The height of the?[M+1]?peak for a particular ion depends on how many carbon atoms are present in that molecule; The more carbon atoms, the larger the?[M+1]?peak is
- For example, the height of the?[M+1]?peak for an hexane (containing six carbon atoms) ion will be greater than the height of the?[M+1]?peak of an ethane (containing two carbon atoms) ion
Worked Example
Determine whether the following mass spectrum belongs to propanal or butanal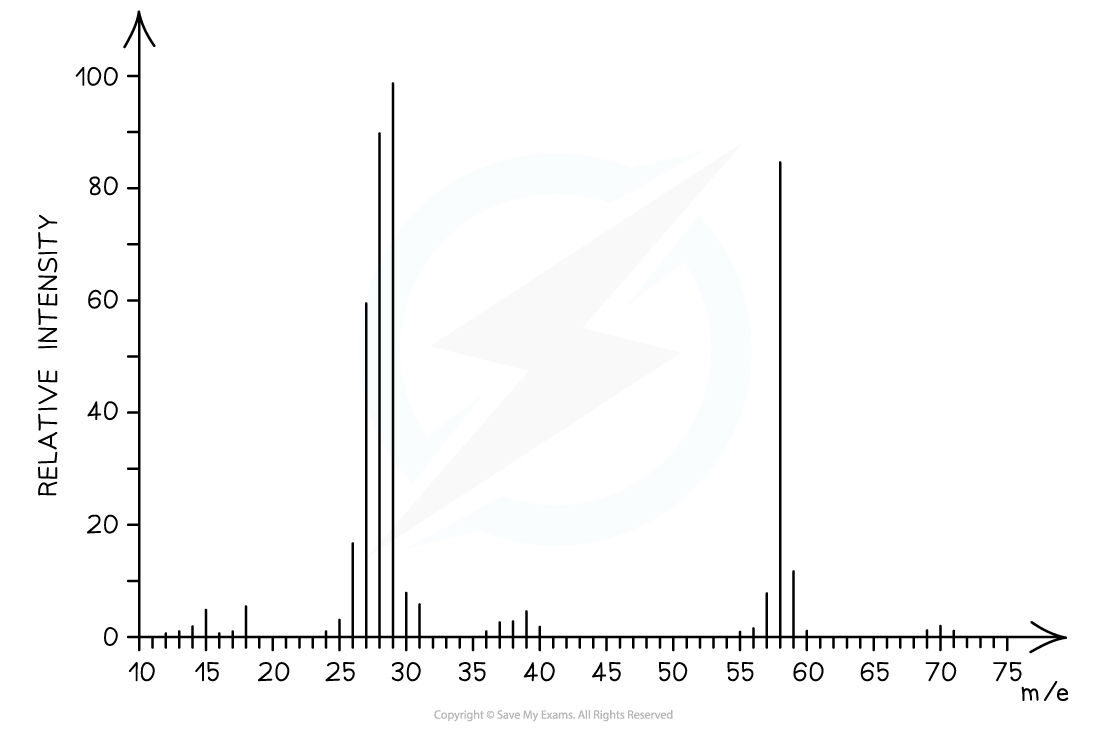
Answer:
-
- The mass spectrum corresponds to?propanal?as the molecular ion peak is at?m/e?= 58
- Propanal arises from the CH3CH2CHO+?ion which has a molecular mass of 58
- Butanal arises from the CH3CH2CH2CHO+?ion which has a molecular mass of 72
Fragmentation Patterns
- The molecular ion peak can be used to identify the?molecular mass?of a compound
- However, different compounds may have the same molecular mass
- To further determine the structure of the unknown compound,?fragmentation analysis?is used
- Fragments may appear due to the formation of?characteristic?fragments?or the?loss?of?small?molecules
- For example, a peak at 29 is due to the characteristic fragment C2H5+--
- Loss of small molecules give rise to peaks at 18 (H2O), 28 (CO), and 44 (CO2)
Alkanes
- Simple alkanes are fragmented in mass spectroscopy by breaking the C-C bonds
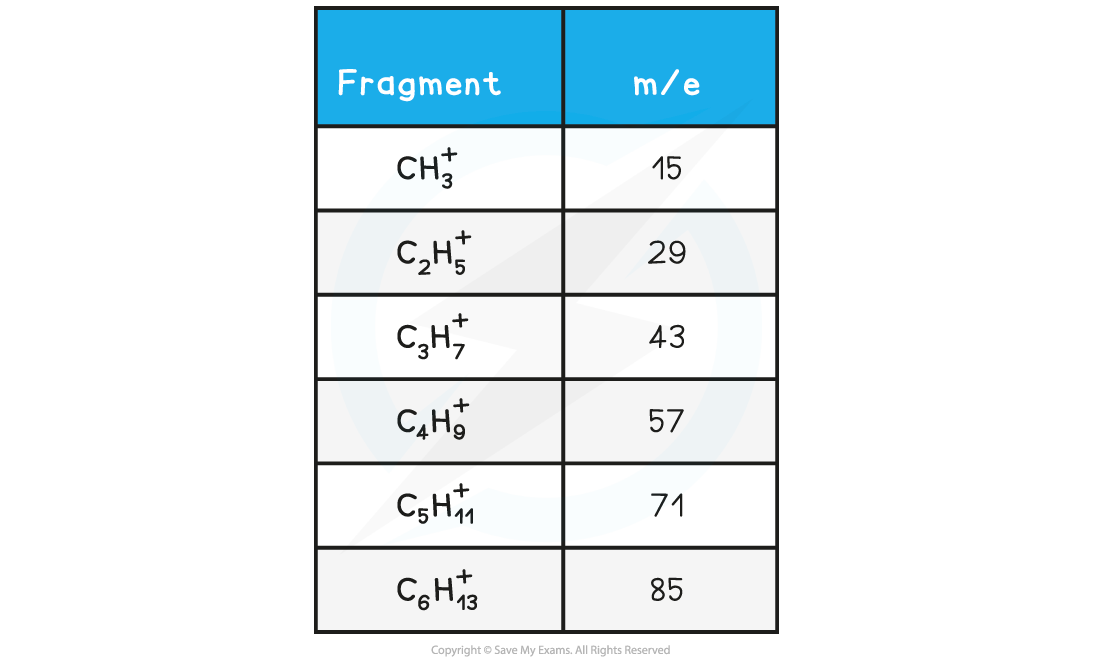
- M/e?values of some of the common alkane fragments are given in the table below
m/e?values of Fragments Table
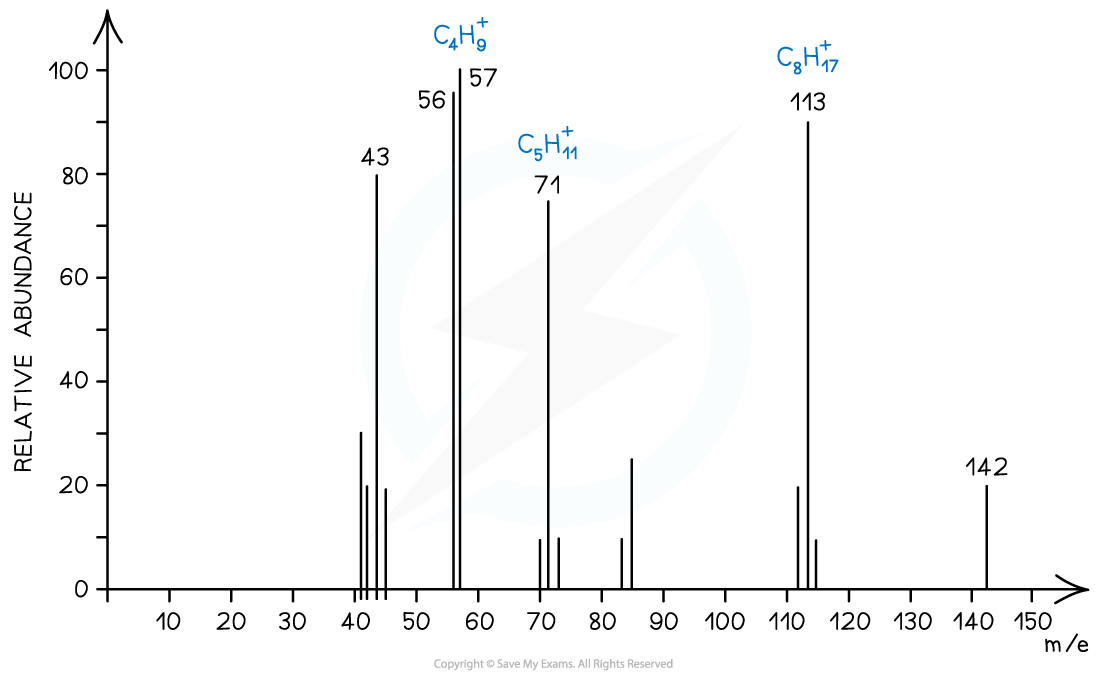
Mass spectrum showing fragmentation of alkanes
Halogenoalkanes
- Halogenoalkanes have often multiple peaks around the molecular ion peak
- This is caused by the fact that there are different isotopes of the halogens
Alcohols
- Alcohols often tend to lose a?water molecule?giving rise to a peak at?18 below the molecular ion
- Another common peak is found at?m/e?value 31 which corresponds to the CH2OH+-- fragment
- For example, the mass spectrum of propan-1-ol shows that the compound has fragmented in four different ways:
- Loss of H to form a C3H7O+?fragment with?m/e?= 59
- Loss of a water molecule to form a C3H6+?fragment with?m/e?= 42
- Loss of a C2H5?to form a CH2OH+?fragment with?m/e?= 31
- And the loss of CH2OH to form a C2H5+?fragment with?m/e?= 29
Exam Tip
A table of mass spectral fragments lost is included in the IB Chemistry Data Booklet Section 28 so you don't need to learn all the likely fragments
轉載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









