- 翰林提供學(xué)術(shù)活動(dòng)、國際課程、科研項(xiàng)目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
Edexcel IGCSE Chemistry 復(fù)習(xí)筆記 4.4.2 Bromine & Alkenes
Edexcel IGCSE Chemistry 復(fù)習(xí)筆記 4.4.2 Bromine & Alkenes
Bromine & Alkenes
- Alkenes are a homologous series of hydrocarbon compounds with at least one?double bond?between two of the carbon atoms on the chain
- The double bond can be written as carbon carbon double bond or as C=C
- The general formula for alkenes is:
CnH2n
- Alkenes are generally more desirable than alkanes as they are?more reactive?due to the presence of the carbon-carbon double bond, so they can take part in reactions in which alkanes cannot, making them more useful than alkanes
- They are used to make?polymers?and are the?starting materials?for the production of many other chemicals
- Two useful reactions are the?bromination of alkenes?and?polymerisation
Bromination of Ethene
- Alkenes undergo addition reactions in which atoms of a simple molecule?add?across?the C=C double bond
- The reaction between bromine and ethene is an example of an addition reaction
- The same process works for any?halogen?and?any alkene?in which the halogen atoms always add to the carbon atoms across the C=C double bond

Bromine atoms add across the C=C in the addition reaction of ethene and bromine
Testing for Alkenes
Bromine Water Test
- Alkanes and alkenes have different molecular structures
- All alkanes are?saturated?and alkenes are?unsaturated
- The presence of the C=C double bond allows alkenes to react in ways that alkanes cannot
- This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine water test
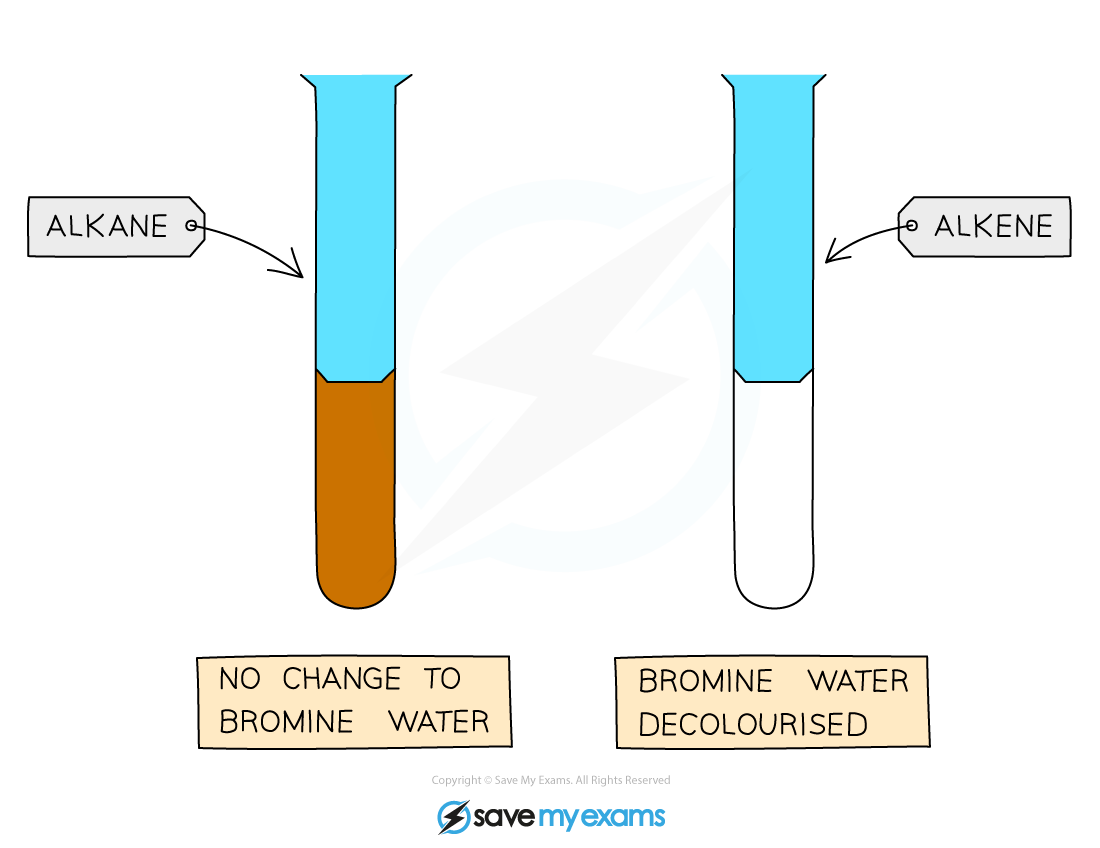
Diagram showing the result of the test using bromine water with alkanes and alkenes
- Bromine water is an?orange?coloured solution
- When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (C=C) so the bromine remains in solution
- But when bromine water is added to an alkene, the bromine atoms add across the C=C bond, hence the solution no longer contains free bromine so it loses its colour
Exam Tip
Alkenes are more reactive than alkanes due to the presence of the carbon-carbon double bond which contains an area of high electron density.
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號(hào)-1









