- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
IB DP Biology: SL復習筆記2.1.1 Molecules
Molecular Biology
The substances of life
- There are?118 elements?in the Periodic Table
- Only the first?92 elements occur in Nature?; the rest are artificially-synthesised in laboratories and are very unstable
- Only around?21 elements are required for life
- The rest have?no role?in sustaining life (some are poisonous eg. arsenic)
- Some elements can be used in?medicine?eg. titanium for skeletal implants, thanks to its inertness, lightness and strength
- There are?4 ubiquitous elements?in biological systems (this means they are found everywhere)
- These 4 make up over 96% of living matter
- Oxygen - 65% of body mass (humans)
- Carbon - 18%
- Hydrogen - 10%
- Nitrogen - 3%
- Other?trace elements?found in organic compounds are: bromine, calcium, chlorine, chromium, copper, iodine, iron, magnesium, manganese, molybdenum, phosphorus, potassium, selenium, silicon and sodium
- There are?other trace elements found in certain phyla only?e.g.?strontium?in certain corals (Cnidaria)
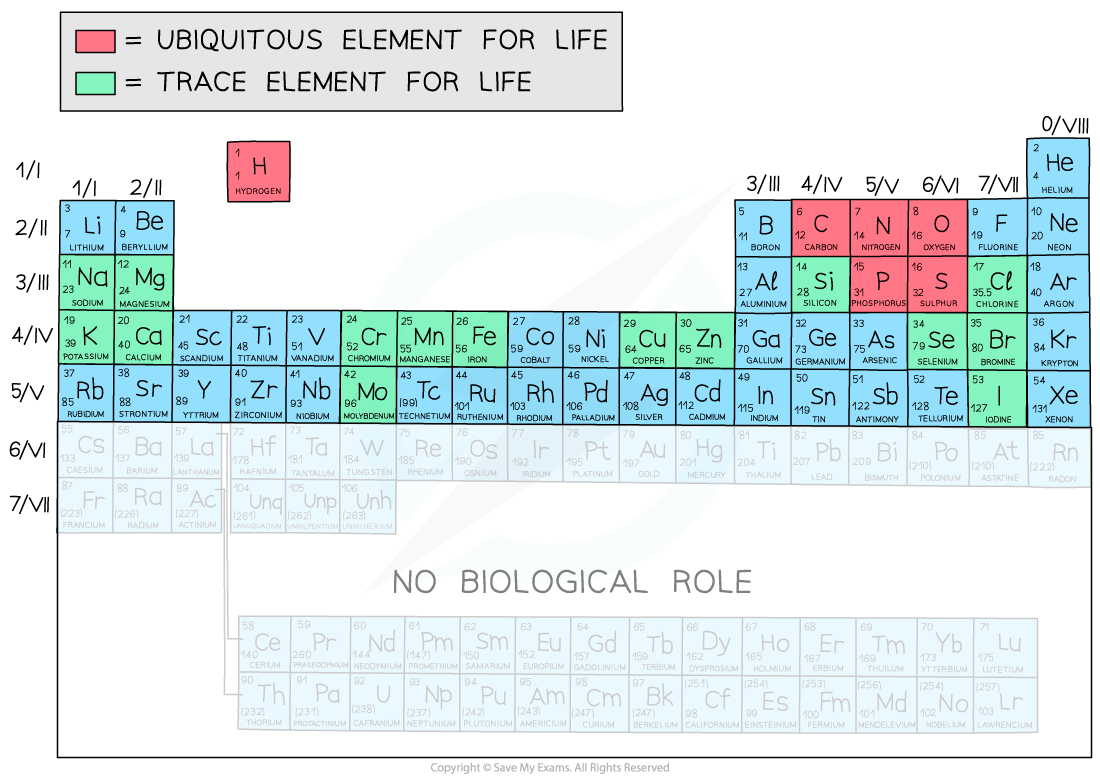
The elements of the Periodic Table that form parts of biological molecules
Elements in biology exist mainly in compounds
- Such compounds are?mainly covalent?compounds
- Electrons are shared?between atoms to generate?strong bonds?within compounds
- For example, elemental carbon only exists as graphite and diamond, which are of no direct use to organisms
- Carbon forms?millions of different covalently-bonded compounds, mainly with hydrogen and oxygen
- Oxygen?is absorbed in elemental form but is quickly converted to its compounds during?transportation?and?respiration
- Some are?ionic?eg. sodium chloride
- Some elements form?prosthetic groups?with larger organic molecules eg. magnesium in chlorophyll, iron in haemoglobin
All of Biology can be explained at a molecular level
- The molecules in cells, and the elements that go to form them, are?the basis of all events?that occur in Nature
- Everything that is observed has a?molecular explanation
- Imagine an all-powerful 'zoom lens' that could look into any level of detail of life
- Such a lens could start at its most zoomed-out, looking at our?biosphere, the Earth
- We assume that alien life does not exist because we haven't found evidence for it yet
- We see?habitats,?populations,?communities?and?individual organisms?coming into view in that order, as we zoom in
- As we zoom in on one organism, we?enter its body?and see increasing levels of detail, right down to the molecular level
- The zoom model helps understand the?important interfaces?between chemistry, biology and physics
- We could zoom in further, to look at sub-atomic particles, although that begins to enter into the realms of physics!
We can zoom into any part of the biosphere to identify all of Biology at a molecular (and atomic) level
Exam Tip
Please note that you do not need to know the specific details of the Periodic Table, it is provided here for context to support your understanding of important biological compounds
Synthesis of Organic Molecules
NOS: Falsification of theories; the artificial synthesis of urea helped to falsify vitalism
- When scientists do not have all the necessary information to understand or explain a biological process/phenomenon they use their knowledge and expertise to propose a?theory
- Other scientists or researchers will often test theories through experiments and gathering data. Sometimes this can lead to an existing theory being disproved or replaced
- In the early 1800s, the theory of?vitalism?stated that a?living force, a mysterious non-molecular entity, was necessary for the synthesis of all?organic molecules
- This theory advocated that all biological molecules were?exclusive to living beings?and could not be found in other branches of science
- Frederick W?hler, a German physician, was the first to synthesise a biological molecule,?urea, from inorganic compounds
- Urea was?thought to be synthesised only?in living organisms
- W?hler heated ammonium cyanate and produced urea, a well-known organic constituent of blood and urine
- Urea had been thought to be found only in living organisms
- The formation of urea from ammonium cyanate?helped to disprove the theory of vitalism, which has been completely?falsified?by subsequent findings
- All of the observations of biology now have a?molecular explanation, and that is now universally accepted
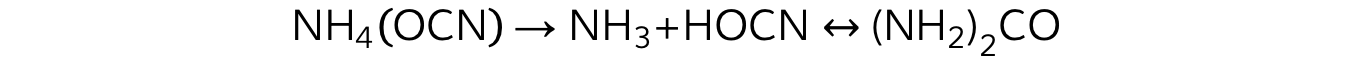
A balanced chemical equation showing the formation of urea

Chemical Structure of Urea
Carbon
- Carbon's unique chemistry makes it the ideal basis of living systems
- The?structure and bonding?possibilities of?carbon?can be detailed as follows:
-
- Four electrons?in its outer (second) shell
- Each atom can form four strong?covalent bonds?using these 4 electrons and therefore forms very?stable, large molecules
-
- Bonds to?other carbon atoms, or?other atoms?such as hydrogen, nitrogen, oxygen, sulfur and the halogens
- Forms?long-chain?and?cyclic?compounds that are stable, this allows a?very high number of possible organic compounds?to exist
- Produce a?tetrahedral structure, due to the four bonds, which allows the formation of varied carbon compounds which have different 3-D shapes and hence, different biological properties
- Double?and?triple bonds?can form with an adjacent carbon atom, allowing?unsaturated?compounds to form
- Can form part of (and join onto) many different?functional groups?that give organic compounds their individual properties
- Alcohol groups
- Hydroxyl groups
- Ketone groups
- Aldehyde groups
- Carbonyl groups
- Amino groups
- Sulfhydryl groups
- Phosphate groups
Carbon Compounds
- The key molecules that are required to build structures that enable organisms to function are:
- Carbohydrates
- Proteins
- Lipids
- Nucleic Acids
- Water
- All of these except water contain carbon
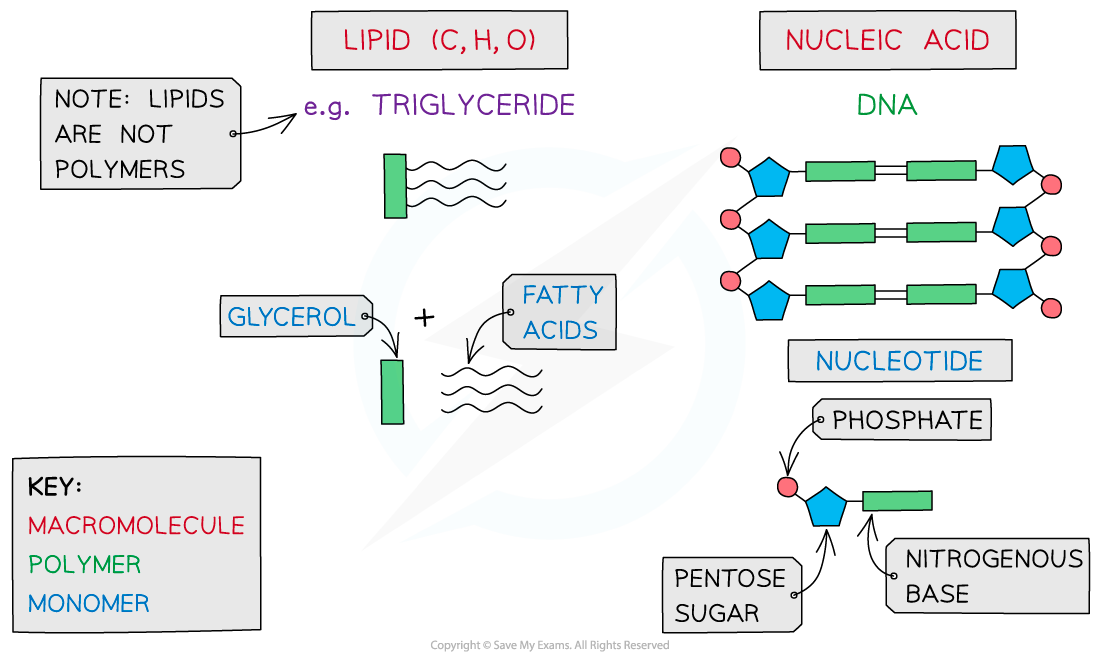
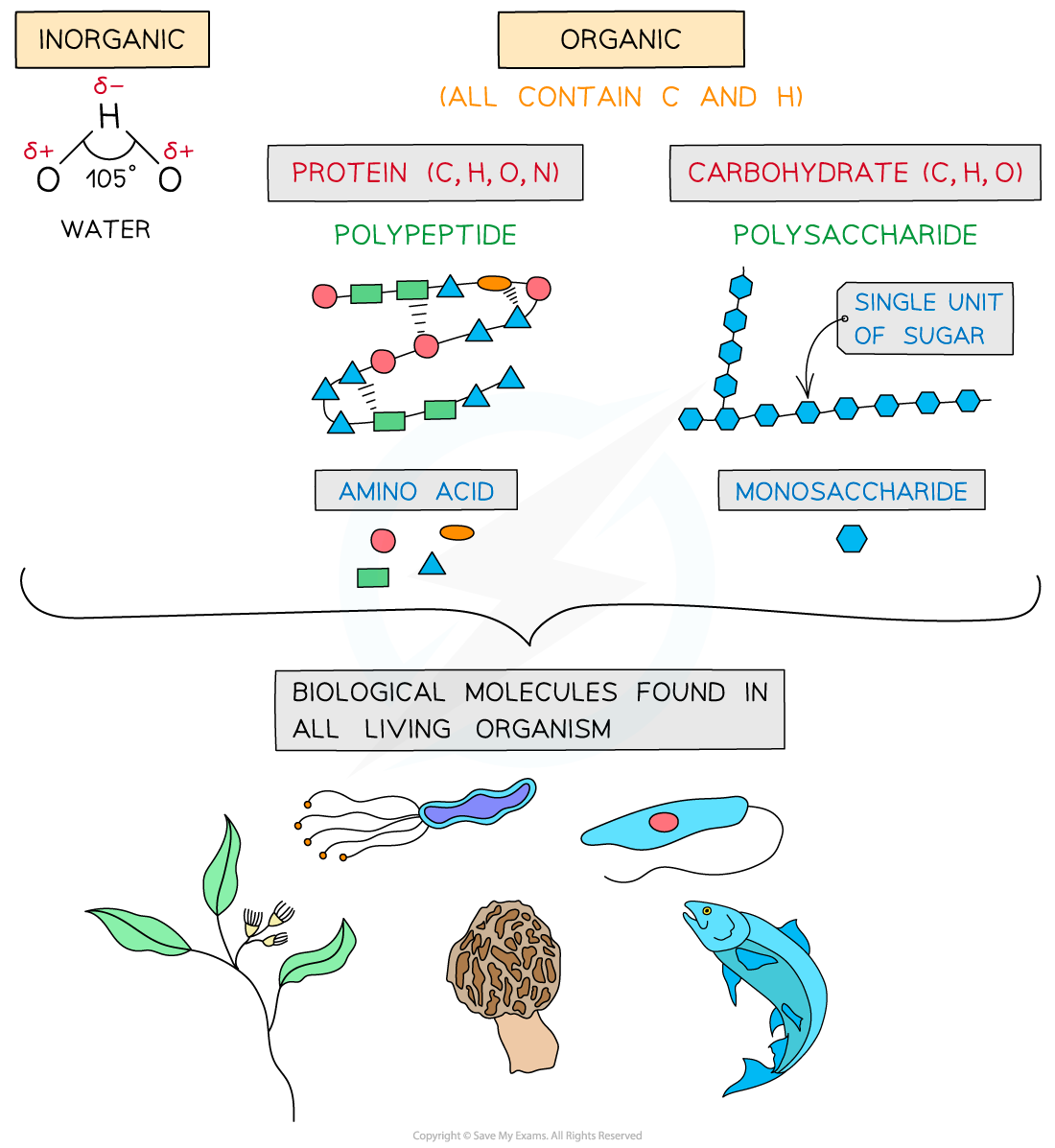
The key biological molecules for living organisms
- Carbohydrates, proteins, lipids and nucleic acids contain the elements?carbon?(C) and?hydrogen?(H) making them?organic compounds
- Carbon atoms are key to organic compounds because:
- Each carbon atom can form?four?covalent bonds?– this makes the compounds very stable (as covalent bonds are so strong they require a large input of energy to break them)
- Carbon atoms can form covalent bonds with oxygen, hydrogen, nitrogen and sulfur
- Carbon atoms can bond to form?straight chains,?branched?chains?or?rings
- Carbon compounds can form small single subunits?(monomers)?that bond with many repeating subunits to form large molecules?(polymers)?by a process called?polymerisation
- Macromolecules?are very large molecules that contain 1000 or more atoms therefore having a?high molecular mass
- Polymers can be macromolecules, however?not?all?macromolecules are polymers as the subunits of polymers have to be the?same?repeating units
Exam Tip
When discussing monomers and polymers, you should be able to give the definition and also name specific examples eg.?nucleic acids?– the?monomer?is a?nucleotide.
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









